Vitamin D PTH and calcium and tumor. Functions of the Skeletal System.

Calcium Foods Functions How Much Do You Need More Eufic
Vital organs are protected by the skeletal system the brain is protected by the skull and the lungs and heart are protected by the rib cage.

. Vitamin D PTH and calcium in relation to survival following prostate cancer. Other types of calcium in supplements and foods include calcium lactate calcium. As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air.
Brandstedt J Almquist M Manjer J Malm J. The skeletal system provides a framework for the body to give it shape. Today we obtain calcium through the electrolysis of a fused salt such as calcium chloride.
Calcium is sometimes referred to as lime. Check the label for the exact amount. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL.
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca 2It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with waterIt has many names including hydrated lime caustic lime builders lime slaked lime cal and pickling limeCalcium hydroxide is used in many. It is the fifth most abundant element in Earths crust and the third most abundant. Calcium carbonate is found in over-the-counter antacid products such as Rolaids or Tums.
Health effects of calcium. Calcium is a chemical element with the symbol Ca and atomic number 20. And ensure that you can take calcium phosphate safely notify your doctor if you have any of the following conditions.
As the divalent cation Ca 2 it is required for structural roles in the cell wall and membranes as a countercation for inorganic and organic anions in the vacuole and as an intracellular messenger in the cytosol Marschner 1995Calcium deficiency is rare in nature but excessive Ca restricts plant communities on. Increased absorption of calcium in the kidneys and intestines. It can be stored in either the liver or adipose tissue.
The physiological functions of calcium are so vital to survival that the body will stimulate bone resorption. Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL. Functions of Cement Ingredients.
A history of kidney stones. How do you make calcium phosphate fertilizer. Vitamin E is a family containing various chemicals including anti-oxidants.
Parathyroid glands are endocrine glands on the posterior surface of the thyroid gland. It was first isolated in 1808 in England when Sir Humphry Davy electrolyzed a mixture of lime and mercuric oxide. Brandstedt J Almquist M Ulmert D Manjer J Malm J.
The following image is showing the ingredients of cement. The main features of these cement ingredients. Calcium is the most abundand metal in the human body.
Calcium phosphate functions better when its taken with food. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. Once exposed to air.
Calcium chromate solubility is 170 gL and at 0 o C calcium hypo chlorate solubility is 218 gL. Calcium acetate is used to control phosphate levels to keep them from getting too high in people with kidney failure who are on dialysis. Calcium is a mineral that is needed for many functions of the body especially bone formation and maintenance.
It is an essential component for the preservation. Calcium is an essential plant nutrient. Calcium carbonate is less expensive.
The general percentage of these ingredients in cement is given below. Calcium was named after the Latin term calx meaning lime and is a reactive silvery metallic element found in Group 2 of the periodic table. Ligaments attach bones to muscles.
Increased mobilisation of calcium from bone activating osteoclasts to release more calcium. It is most commonly found in milk and milk products but also in vegetables nuts and beans. Method of producing calcium phosphate fertilizer from a calcium-containing material reactive with diluted phosphoric acid.
Calcium Ca 2 is a divalent cation that plays a critical role in numerous body functions such as skeletal mineralization signal transduction nerve conduction muscle contraction and blood coagulationCa 2 metabolism is linked to magnesium Mg 2 and phosphate metabolismCa 2 homeostasis is dependent on intestinal absorption bone. Parathyroid glands play a vital role in regulating the. It is absorbed better by the body if taken with food.
Calcium can also bind to other minerals such as phosphate and aid in their removal from the body. Long bones produce red. Is the main constituent of bones and theets and it has keys metabolic functions.
Each chew or pill usually provides 200 to 400 mg of calcium.
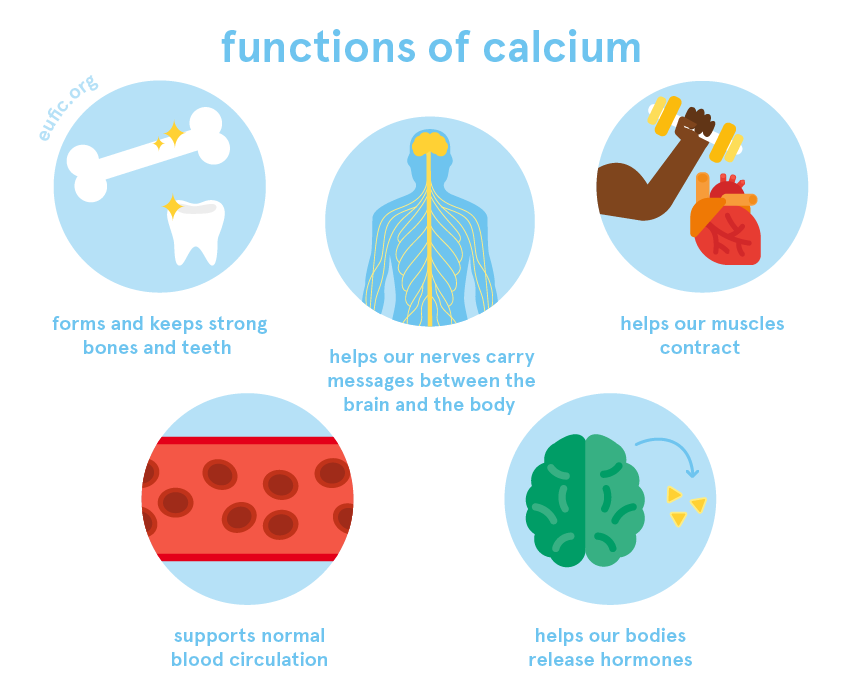
Calcium Foods Functions How Much Do You Need More Eufic


0 Comments